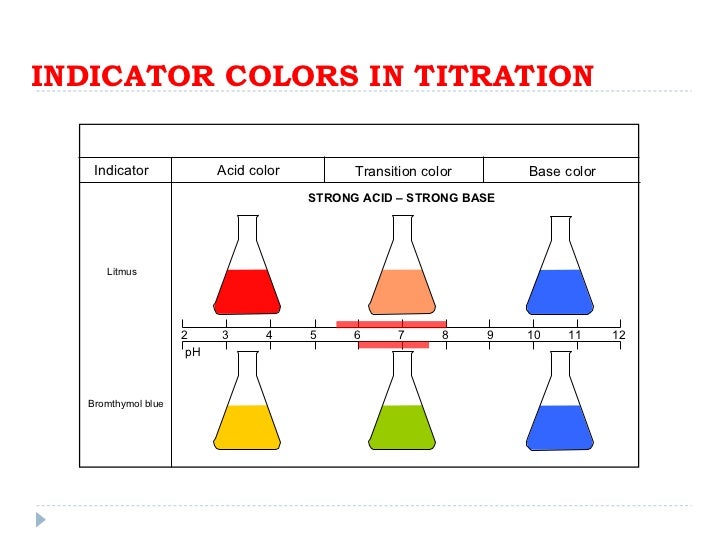
Indicators For Titrations . The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the. The weak acid titration curve in figure \(\pageindex{3}\) shows that only one of the three indicators is suitable for end point detection. The titration curves shown in figure 14.20 illustrate the choice of a suitable indicator for specific titrations. In the strong acid titration,. Remember that the equivalence point of a titration is where you have mixed the two substances in exactly. The two most common indicators that are used in titrations are methyl orange and phenolphthalein. The ph range of phenolphthalein is about 8.3 to 10.0, but the. This page assumes that you know.
from mungfali.com
The titration curves shown in figure 14.20 illustrate the choice of a suitable indicator for specific titrations. The two most common indicators that are used in titrations are methyl orange and phenolphthalein. Remember that the equivalence point of a titration is where you have mixed the two substances in exactly. The weak acid titration curve in figure \(\pageindex{3}\) shows that only one of the three indicators is suitable for end point detection. The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the. In the strong acid titration,. The ph range of phenolphthalein is about 8.3 to 10.0, but the. This page assumes that you know.
Acid Base Titration Indicator
Indicators For Titrations The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the. The ph range of phenolphthalein is about 8.3 to 10.0, but the. In the strong acid titration,. The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the. The two most common indicators that are used in titrations are methyl orange and phenolphthalein. Remember that the equivalence point of a titration is where you have mixed the two substances in exactly. This page assumes that you know. The titration curves shown in figure 14.20 illustrate the choice of a suitable indicator for specific titrations. The weak acid titration curve in figure \(\pageindex{3}\) shows that only one of the three indicators is suitable for end point detection.
From www.youtube.com
An Introduction To Titration Calculations (GCSE Chemistry) YouTube Indicators For Titrations The titration curves shown in figure 14.20 illustrate the choice of a suitable indicator for specific titrations. The ph range of phenolphthalein is about 8.3 to 10.0, but the. The two most common indicators that are used in titrations are methyl orange and phenolphthalein. This page assumes that you know. The weak acid titration curve in figure \(\pageindex{3}\) shows that. Indicators For Titrations.
From themasterchemistry.com
Acid Base TitrationWorking Principle, Process, Types And Indicators Indicators For Titrations The weak acid titration curve in figure \(\pageindex{3}\) shows that only one of the three indicators is suitable for end point detection. The titration curves shown in figure 14.20 illustrate the choice of a suitable indicator for specific titrations. In the strong acid titration,. The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the. The. Indicators For Titrations.
From classnotes.org.in
Acid Base Titration using Indicator Chemistry, Class 11, Ionic Indicators For Titrations The titration curves shown in figure 14.20 illustrate the choice of a suitable indicator for specific titrations. The weak acid titration curve in figure \(\pageindex{3}\) shows that only one of the three indicators is suitable for end point detection. The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the. In the strong acid titration,. The. Indicators For Titrations.
From psu.pb.unizin.org
14.7 AcidBase Titrations Chemistry 112 Chapters 1217 of OpenStax Indicators For Titrations The weak acid titration curve in figure \(\pageindex{3}\) shows that only one of the three indicators is suitable for end point detection. The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the. The two most common indicators that are used in titrations are methyl orange and phenolphthalein. Remember that the equivalence point of a titration. Indicators For Titrations.
From solutionpharmacy.in
Complexometric Titration Solution Parmacy Indicators For Titrations This page assumes that you know. The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the. The titration curves shown in figure 14.20 illustrate the choice of a suitable indicator for specific titrations. Remember that the equivalence point of a titration is where you have mixed the two substances in exactly. In the strong acid. Indicators For Titrations.
From www.clipartkey.com
Redox Titration Apparatus Of Ferrous Ions By Ceric Redox Indicators Indicators For Titrations This page assumes that you know. The ph range of phenolphthalein is about 8.3 to 10.0, but the. Remember that the equivalence point of a titration is where you have mixed the two substances in exactly. In the strong acid titration,. The titration curves shown in figure 14.20 illustrate the choice of a suitable indicator for specific titrations. The two. Indicators For Titrations.
From www.slideserve.com
PPT Titrations PowerPoint Presentation, free download ID5572517 Indicators For Titrations In the strong acid titration,. The weak acid titration curve in figure \(\pageindex{3}\) shows that only one of the three indicators is suitable for end point detection. The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the. This page assumes that you know. The two most common indicators that are used in titrations are methyl. Indicators For Titrations.
From chrominfo.blogspot.com
Chrominfo Indicators of complexometric titration Indicators For Titrations The two most common indicators that are used in titrations are methyl orange and phenolphthalein. The weak acid titration curve in figure \(\pageindex{3}\) shows that only one of the three indicators is suitable for end point detection. This page assumes that you know. The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the. In the. Indicators For Titrations.
From mmerevise.co.uk
Titrations and Uncertainties MME Indicators For Titrations This page assumes that you know. The weak acid titration curve in figure \(\pageindex{3}\) shows that only one of the three indicators is suitable for end point detection. The two most common indicators that are used in titrations are methyl orange and phenolphthalein. The titration curves shown in figure 14.20 illustrate the choice of a suitable indicator for specific titrations.. Indicators For Titrations.
From www.slideserve.com
PPT Acid Base Titrations PowerPoint Presentation ID2145680 Indicators For Titrations The weak acid titration curve in figure \(\pageindex{3}\) shows that only one of the three indicators is suitable for end point detection. The ph range of phenolphthalein is about 8.3 to 10.0, but the. This page assumes that you know. Remember that the equivalence point of a titration is where you have mixed the two substances in exactly. In the. Indicators For Titrations.
From www.compoundchem.com
Compound Interest Chemistry Techniques Titration Indicators For Titrations In the strong acid titration,. This page assumes that you know. The titration curves shown in figure 14.20 illustrate the choice of a suitable indicator for specific titrations. The weak acid titration curve in figure \(\pageindex{3}\) shows that only one of the three indicators is suitable for end point detection. The graph shows the results obtained using two indicators (methyl. Indicators For Titrations.
From www.slideserve.com
PPT Indicators for AcidBase Titrations (Sec. 96) PowerPoint Indicators For Titrations In the strong acid titration,. Remember that the equivalence point of a titration is where you have mixed the two substances in exactly. The two most common indicators that are used in titrations are methyl orange and phenolphthalein. This page assumes that you know. The weak acid titration curve in figure \(\pageindex{3}\) shows that only one of the three indicators. Indicators For Titrations.
From www.dreamstime.com
Phenolphthalein Indicator in Acidbase Titration Stock Vector Indicators For Titrations The ph range of phenolphthalein is about 8.3 to 10.0, but the. The titration curves shown in figure 14.20 illustrate the choice of a suitable indicator for specific titrations. In the strong acid titration,. The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the. Remember that the equivalence point of a titration is where you. Indicators For Titrations.
From franco-krussell.blogspot.com
How to Determine Which Indicator to Use for Titration Indicators For Titrations The ph range of phenolphthalein is about 8.3 to 10.0, but the. The weak acid titration curve in figure \(\pageindex{3}\) shows that only one of the three indicators is suitable for end point detection. The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the. The two most common indicators that are used in titrations are. Indicators For Titrations.
From www.science-revision.co.uk
Titrations Indicators For Titrations The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the. Remember that the equivalence point of a titration is where you have mixed the two substances in exactly. The ph range of phenolphthalein is about 8.3 to 10.0, but the. The two most common indicators that are used in titrations are methyl orange and phenolphthalein.. Indicators For Titrations.
From mungfali.com
Acid Base Titration Indicator Indicators For Titrations The weak acid titration curve in figure \(\pageindex{3}\) shows that only one of the three indicators is suitable for end point detection. The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the. This page assumes that you know. Remember that the equivalence point of a titration is where you have mixed the two substances in. Indicators For Titrations.
From www.thesciencehive.co.uk
Acids, Alkalis and Titrations (GCSE) — the science hive Indicators For Titrations Remember that the equivalence point of a titration is where you have mixed the two substances in exactly. In the strong acid titration,. The weak acid titration curve in figure \(\pageindex{3}\) shows that only one of the three indicators is suitable for end point detection. This page assumes that you know. The two most common indicators that are used in. Indicators For Titrations.
From mungfali.com
Acid Base Titration Indicator Indicators For Titrations The weak acid titration curve in figure \(\pageindex{3}\) shows that only one of the three indicators is suitable for end point detection. In the strong acid titration,. The titration curves shown in figure 14.20 illustrate the choice of a suitable indicator for specific titrations. The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the. This. Indicators For Titrations.